Describe the Bonding in F-xe-f Using Mo Theory
It has a distance of 195 A between the Xe and F. We must first draw the Lewis structure for XeF₂.
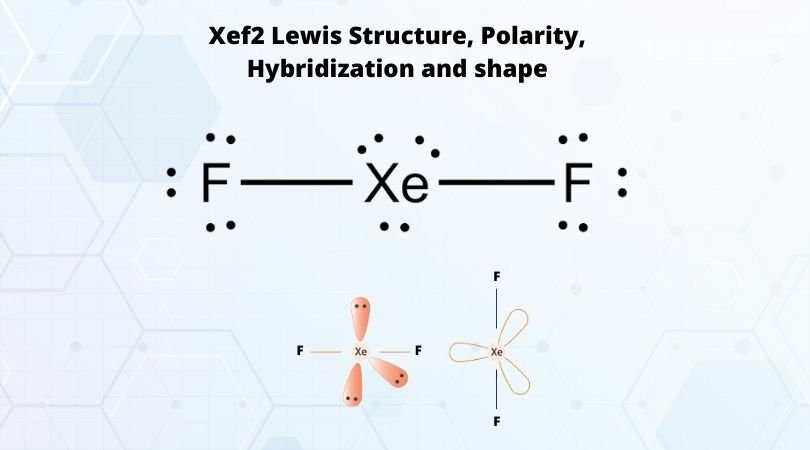
Xef2 Lewis Structure Polarity Hybridization And Shape
Chemistry questions and answers.

. According to VB theory a covalent bond forms from the physical overlap of. Addition of AOs forms a bonding MO which has a region of high electron density between the nuclei. Valence bond theory explains the number of bonds formed in a compound and the relative bond strengths.
Subtraction of AOs forms an antibonding MO which has. Because arguments based on atomic orbitals focus on the bonds formed between valence electrons on an atom they are often said to involve a valence-bond theory. Use MO theory to describe the bonding in each of these diatomic molecules.
Therefore in 1932 F. The VSEPR model states that the electron regions around an atom spread out to make each region is as far from. A Draw an MO energy diagram B Predict if the molecule is paramagnetic or diamagnetic C Determine the bond order D Predict if adding an additional electron would strengthen or weaken the bond E Predict if.
Take note that these are theories and should be treated as such. Not allowed around double bonds rotation disturbs the parallel alignment of the p-orbitals and reduces their overlap the π-bond breaks Molecules with double bonds can have cis-trans isomers Example. The valence-bond model cant adequately explain the fact that some molecules contains two equivalent bonds with a bond order between that of a single bond and.
Possibly unaware that Lewiss model existed Walter Heitler and Fritz London. In other words there is a single bond connecting the two H atoms in the H 2 molecule. It will require six orbitals to describe the bonding.
In NH 3 for example N with a 2 s2 2 p3 valence electron configuration can hybridize its 2. The combination of two atomic orbitals always forms two molecular orbitals. 0 Three lone pairs in equatorial hybrids EPG 6 pairs d 2 sp 3 hybrids four σ bonds at 90 0 in a plane Two lone pairs in axial hybrids Describe the molecular structure and bonding in XeF 2 and XeF 4 Linear Xe F F F F Square planar.
The bonding in XeF3- is qualitatively described by using molecular orbital approaches using either a diradical approach or orbital interaction models. This tells us that there are five electron regions Steric Number 5 about the central carbon atom. It is one of the two common theories that helps describe the bonding between atoms.
The bonding in molecules such as NH 3 or H 2 O which have lone pairs on the central atom can also be described in terms of hybrid atomic orbitals. These bond angles contribute to the formation of square planar molecular geometry. F 2porbitals in F2 the H 1sand F 2porbitals in HF and the Cl 3p orbital and Br 4porbital in ClBr.
Learn VSEPR Theory to know the geometrical arrangement of various. The bond order for a molecule can be determined as follows. KrF2 would also be expected on the basis of the VSEPR approach to have single KrF bonds in addition to three lone pairs on Kr.
Describe the bonding in XeF4 using hybrid orbitals. The xenon atom has four single bonds and two lone pairs. The VSEPR theory predicts that XeF₂ is linear.
Molecular orbitals wave functions result from adding andor subtracting atomic orbitals wave functions. In chemistry valence bond VB theory is one of two basic theoriesalong with molecular orbital MO theorythat use quantum mechanics to explain chemical bonding. B2 C2 N2 O2 F2 CO NO For each.
The bond angles of F-Xe-F are 90 degrees and lone pairs have angles of 180 degrees. The bonding molecular orbital which is _____ in energy and the antibonding molecular orbital which is _____ in energy than the original atomic orbitals. Valence Bond VB Theory looks at the interaction between atoms to explain chemical bonds.
After the hybridization takes place 4 number of half-filled orbitals form covalent bonds with the half-filled 2p orbitals of the fluorine atoms in the structure. The single bond present in each molecule results from overlap of the relevant orbitals. They are not always perfect.
As there are fluorine molecules on both the side of the central atom there is no dipole moment and hence there is no polarity. Bond order ½ bonding electrons antibonding electrons. It also explains the bonding in a number of other molecules such as violations of the octet rule and more molecules with more complicated bonding beyond the scope of this text that are difficult to describe with Lewis structures.
Sketch the overlap of the atomic orbitals involved in the bonds. This suggests that you use d2sp3 hybrid orbitals on xenon. MO theory tells us that both electrons are in the mathsigma math bonding orbital 50 of which comes from one atom lets call it A and 50 from the other lets call it B.
The Fluorine atoms are located at 90 degrees to each other resulting in the symmetric distribution of the electrons in the molecules plane. Atomic wave functions AOs combine to form molecular wave functions MOs. If we therefore consider the case of a single electron we find that it is.
EXAMPLES Xe F F EPG 5 pairs dsp 3. They are the three lone pairs and the two Xe-F bonds. COMPARISM BETWEEN VALENCE BOND VB THEORY CRYSTAL FIELD THEORY CFT AND MOLECULAR ORBITAL MO THEORY OF COORDINATION COMPOUNDS.
Use valence bond theory to explain the bonding in F2 HF and ClBr. Therefore the H 2 molecule has a bond order of ½ 2 0 1. A covalent bond forms between the two atoms by the overlap of half-filled valence atomic orbitals from each atom.
XeF2 is nonpolar due to the symmetric arrangement of the bonded pairs of electrons. Mulliken came up with Molecular Orbital Theory to explain questions like the ones above. INTRODUCTION Valence Bond Theory has its roots in Gilbert Newton Lewiss paper The Atom and The Molecule.
Molecular Orbital Theory. The other theories is the Molecular Orbital Theory. A Problem to Consider Describe the bonding in XeF4 using hybrid orbitals.
Molecular Orbital MO Theory The combination of orbitals to form bonds is viewed as the combination of wave functions. In the case of He 2 on the other hand the bond order is ½ 2 2 0. This is particularly explained by the valence bond theory as the two non-bonding electrons from 5p are promoted to the 5d orbital.
Valence Bond Model vs. The bond angle of F-Xe-F is 180 degrees. 96 Molecular Orbital Theory Molecular Orbital Theory MO Atomic orbitals combine to form new molecular orbitals which are spread out over the entire molecule.
MOLECULAR ORBITAL THEORY electrons occupy orbitals each of which spans the. Two low-energy singlet structures are identified for XeF3- consisting of Y- and T-shaped geometries and there is. C2H2Cl2 113 Molecular Orbital MO Theory Limitations of the VSEPR model and the VB theory based on localized bonding e-pairs.
511 The molecular orbital description of KrF would predict that this ion which has the same number of valence electrons as F2 would have a single bond. Molecular orbital theory MO theory provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. A molecular orbital is a region of space in a covalent species where electrons are likely to be found.
Each Xe-F bond is formed by. Electrons are in orbitals that belong to the molecule as a whole. The Valence Bond Theory fails to answer certain questions like why He 2 molecule does not exist and why O 2 is paramagnetic.

Vb Mixing Diagrams For The 3c 4e Bond In F Xe F The Energies Of The Download Scientific Diagram

Xef2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
No comments for "Describe the Bonding in F-xe-f Using Mo Theory"
Post a Comment